Chronic kidney disease (CKD) is an ongoing decline of renal function which may progress quite rapidly or develop slowly over many years. Due to the nature of the disease, it is linked to cardiovascular disease (CVD) and a host of symptoms, comorbidities, high mortality and reduced quality of life. The majority of CKD patients will be identified in primary care. Once diagnosed, patients are likely to become increasingly frequent attenders within general practice. It is therefore useful to have an overview of the disease process and the multifaceted care needs of this specific patient group.
FACTS AND FIGURES
The kidneys are a pair of fist-sized organs situated in the lower back. Each kidney contains approximately 1,000,000 filtering units known as nephrons. Over the course of 24 hours, healthy kidneys filter approximately 180 litres of plasma to extract waste, toxins and excess water. This is all excreted via the production of approximately two litres of urine per day, which drains continually to the bladder via the ureters (Kriz and Elger, 2024). However, urine production is just one of the many roles of this multitasking organ. The kidney also secretes erythropoietin and regulates blood pressure, bone metabolism, acid-base balance, fluid control and electrolyte balance. Unfortunately, when the kidneys fail, so do all these vital processes leading to serious conditions such as renal anaemia, cardiovascular disease (CVD), mineral bone disorder, metabolic acidosis and hyperkalaemia, to name but a few (Bailey and Unwin, 2024).The single most common cause of chronic kidney disease (CKD) in the UK is diabetes. In 2021, 31.3% of adults commencing renal replacement therapy, or RRT (i.e. dialysis or kidney transplantation), had diabetes as the primary cause of renal failure. This was followed by glomerulonephritis as the primary cause at 13.3% (diseases which destroy the filtering process) (UK Renal Registry, 2023). Other primary causes were hypertension at 6.8%, pyelonephritis (kidney infection) at 4.9%, polycystic kidney disease (6.4%), and renal vascular disease (4.6%). Other causes, including nephrotoxic agents (e.g. non-steroidal anti-inflammatory drugs [NSAIDs], such as ibuprofen or diclofenac), structural renal tract impairment (e.g. malignancy or enlarged prostate) and recurrent urinary stones were 17.6% (UK Renal Registry, 2023). However, the actual cause of CKD can frequently be unknown (as was the case for 15.1% of patients starting RRT in 2021) (UK Renal Registry, 2023).
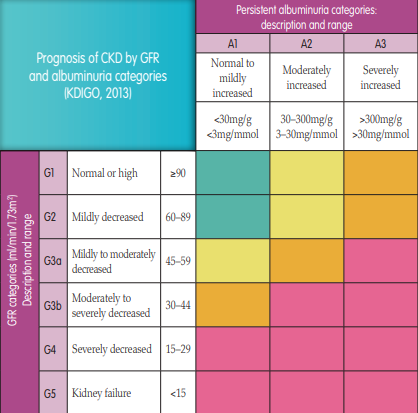
CKD describes kidneys which have lost their normal level of function for longer than 90 days (Kidney Disease Improving Global Outcomes [KDIGO] guidelines, 2013). It is a common, progressive and life-long illness which is categorised into five stages (Figure 1). CKD is incurable and can be difficult to spot in the early stages when the patient is often asymptomatic. In fact, symptoms may only present when two-thirds of the total kidney function has already been lost. Due to the various bodily processes controlled by the kidney, multiple health issues such as those mentioned above can gradually develop and worsen. The additional symptoms of uraemia, such as tiredness, headaches, itching, nausea, vomiting, oedema and loss of appetite can also develop if the patient develops CKD stage 5. In the absence of appropriate management, CKD is potentially life-threatening (KDIGO, 2013).
FIGURE 1.
Prognosis of CKD by glomerular filtration rate (GFR) and albuminuria categories (adapted from KDIGO, 2013). Green = low risk; yellow = moderately increased risk; orange = high risk; pink = very high risk.It is estimated that 3.5 million people in the UK have moderate-to-severe CKD, which is categorised as stages 3 to 5 (Kidney Care UK, 2023). During these mid- to latter-stages, patients are also likely to be living with comorbidities such as diabetes, hypertension and CVD, which will increase in severity as the renal function declines (UK Kidney Association, 2017).
CKD also carries a heavy financial burden. In 2023, the total cost of kidney disease to the UK economy was estimated at £7 billion. This includes £6.4 billion in direct costs to the NHS; approximately 3.2% of the £197 billion total NHS spending across the four nations. It also includes £372 million productivity loss for people living with kidney failure and the people who care for them, and £225 million in dialysis transport costs (Kidney Research UK, 2023).
TESTS TO DIAGNOSE CKD
Indicators of CKD may be identified through blood tests or urinalysis at any time. When interpreting the results of a urinalysis, it is important to remember that the presence of haematuria and/or proteinuria (urinary blood or protein) should not be assumed to solely indicate a urinary tract infection (UTI). This is especially true for a patient with hypertension or urinalysis results with an absence of urinary leucocytes and nitrites (it is therefore important that the reagent stick has indictors for these). Of course, the presence of a UTI should be excluded through the urine sample being sent to the lab for culture. Blood or protein could point to more serious conditions such as an intrinsic renal disease, e.g. glomerulonephritis (Carter et al, 2006).
To confirm CKD, the KDIGO guidelines (2013) recommend that the following two tests are performed on at least two separate occasions, separated by a minimum of 90 days. Either of the following must be present for more than three months for CKD to be diagnosed:
- A GFR of less than 60
- An albumin/creatinine ratio (ACR) of more than 30mg/g, or other markers of kidney damage (see below).
This is calculated from a blood test and is an estimate of how well the blood is being filtered by the kidneys. The formula recommended by NICE (2021a) for use in clinical laboratories is the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation to estimate GFR in adults, using creatinine assays. CKD-EPI generally provides more accurate estimates of GFR, particularly in patients with higher levels of kidney function (i.e. eGFR >60ml/min/1.73 m²).
An eGFR over 90ml/min/1.73m2 of body surface area is considered normal unless there is other evidence of kidney disease (UK Kidney Association, 2017). CKD is indicated by a GFR of less than 60ml/min/1.73m2 persisting for more than three months. This represents less than half the normal level of a healthy young adult. This calculation can be thought of as a percentage of normal kidney function. For example, a GFR of less than 60ml/ min/1.73m2 means that less than 60% of kidney function remains.
Creatinine measurement can alter due to various reasons. Therefore, the blood sample should be taken from a non-fasted patient who should not have eaten meat for 12 hours before the test. Blood should be processed within 12 hours of collection.
It should be noted that the GFR may be less reliable in certain situations (e.g. acute kidney injury [AKI] — previously known as acute renal failure), pregnancy, oedematous states, large muscle mass, malnourished patients or amputees, and has not been well validated in certain ethnic groups (e.g. in people of Asian or African family origin). Also, to avoid AKI being classified as CKD, the serum creatinine should be looked at with caution in patients who have no previous blood results. An eGFR result of less than 60ml/min/1.73 m2 should be confirmed in a person not previously tested by repeating the test within two weeks (NICE, 2021a).
Urine albumin/creatinine ratio (ACR)
Albuminuria describes an increase of albumin excreted within the urine. An increased ACR has been shown to be an independent risk factor associated with diabetes, CKD, CVD, hypertension, venous thromboembolism and all-cause mortality (Mahmoodi et al, 2009). Albumin is a protein present in large amounts in the blood. If the nephrons have been damaged (often seen in diabetes), albumin is able to leak from the blood into the urine and is detected through an ACR test (NHS, 2022a).
This just requires an early morning urine sample to be sent to the lab to calculate the urinary albumin concentration in relation to the dilution of creatinine. It is highly sensitive and detects urinary protein in early disease that could not otherwise be picked up by urinalysis (NICE, 2021a). The ACR provides an estimate of the amount of albumin excreted within 24 hours without the need for a 24-hour urine collection. Urinary excretion of abnormal quantities of albumin for more than three months, with or without a decrease in GFR, is diagnostic of CKD (KDIGO, 2013).
As mentioned above, there are other markers of CKD:
- Urine sediment abnormalities
- Electrolyte and other abnormalities due to tubular disorders
- Abnormalities detected by histology
- Structural abnormalities detected by imaging
- History of kidney transplantation.
CKD AND COMPLICATIONS
There are many complications which arise during the progression of CKD. A couple of examples which can occur fairly early on are renal anaemia and CKD-mineral and bone disorder (CKD-MBD).Anaemia
Renal anaemia is an important and common complication of CKD, plus it is yet another symptom of CKD for the patient to cope with. Reduced circulating oxygen can lead to increased cardiac output, left ventricular hypertrophy, reduced cognition and concentration, reduced libido and reduced immune responsiveness. Severe renal anaemia is uncommon before CKD stage 3b, but it will start to increase in line with declining GFR (UK Kidney Association, 2017). However, anaemia is both potentially reversible and controllable with the correct treatment, which can reduce cardiovascular complications and improve quality of life.
NICE (2021a) recommends that adult CKD patients should be investigated and have their anaemia managed if:
- Their haemoglobin (Hb) level falls to 110g/litre or less
- They develop symptoms attributable to anaemia (such as tiredness, shortness of breath, lethargy and palpitations).
Iron deficiency can be due to a combination of the poor dietary iron-absorption of CKD patients, multiple blood tests, low-grade gastrointestinal bleeding, or for those on dialysis, blood left in the dialysis circuit. Alternatively, the body’s iron stores may be normal, but the supply from the stores is inadequate to sustain the demands of the bone marrow (Wish, 2006). Replacement iron is generally given intravenously rather than orally for patients with CKD. This is a controversial subject, but there is evidence of the haemoglobin response being greater with intravenous versus oral iron (O’Lone, 2019).
Blood transfusions are avoided due to a greater risk of complications, especially for patients who would potentially hope to be considered to receive a donor organ. This is to improve the patient’s suitability and outcomes in kidney transplantation due to reduced human leukocyte antigen (HLA) sensitisation.
CKD-mineral and bone disorder (CKD-MBD)
CKD-MBD is a frequent complication in which the patient’s bones can become thin and weak due to deteriorating renal function. Without treatment, the patient will experience bone pain and an increased risk of fractures. The kidneys play a central role in the homeostasis of calcium and phosphate through the processes of secretion and reabsorption of these ions in the nephrons. Effective absorption of calcium from the gut is also controlled by the kidneys’ ability to convert vitamin D into its most active form (Dring and Hipkiss, 2015).
Low serum calcium levels trigger the release of parathyroid hormone (PTH) from the parathyroid glands, which prompts the bones to release their calcium to achieve homeostasis. Over time, the parathyroid glands will enlarge and excrete greater amounts of PTH than is actually required, causing even more calcium to be leeched from the bones. The serum calcium level is also dictated by the phosphate level. As serum phosphate rises, serum calcium decreases leading to an increase in PTH secretion and further calcium loss from the bones.
In addition to contributing to the weakening of the bones, elevated serum phosphate increases the already heavy burden of CVD in CKD. Raised phosphate levels lead to the deposit of calcium phosphate salts in valvular and vascular tissue. These deposits result in the calcification and stiffening of blood vessels. This greatly increases the patient’s risk of suffering cardiovascular events (Lau et al, 2010).
Some CKD patients may find that a low-phosphate diet alone is sufficient, while others will be prescribed ‘phosphate binder’ medication. This is taken at mealtimes and prevents the absorption of phosphate in the gut. The patient may also be prescribed vitamin D medication in one of its various forms. In some cases, calcimimetic medication may be appropriate. This drug mimics calcium at the PTH receptor, which will then assume that the serum calcium level is normal and further PTH secretion will be reduced (Blaine et al, 2015).
CKD AT STAGE 5
Although CKD will remain stable in many patients, in a growing number it will progress towards end-stage renal failure (CKD stage 5) (Hashmi et al, 2023). These patients are then faced with the decision of what happens next. The renal multidisciplinary team (MDT) can provide support and information to the patient regarding the options of dialysis, transplantation or conservative management.Dialysis
In 2021, just under 69,500 UK adults were on a form of kidney replacement treatment. That year, close to 7,700 people started dialysis. While an effective treatment, dialysis does not fully replace kidney function and does not cure the underlying disease. It may take place by one of two methods, namely haemodialysis or peritoneal dialysis (UK Renal Registry, 2023).
Haemodialysis (HD)
Toxins, waste products and excess fluid are removed from the patient generally three times a week via a haemodialysis machine in hospital over roughly four hours. Should the patient dialyse at home, this is likely to involve five sessions a week. During dialysis, the patient’s blood undergoes a continuous cycle of being removed from the body, passed through a filter and returned to the patient.
Access to the blood may be via a permacath sited in the upper chest or via an arterio-venous fistula (AVF). An AVF is the surgical connection of an artery to a vein and is generally in the patient’s arm. Blood from the artery then travels directly into that vein, causing it to become bigger and firmer. In some cases, an artificial tube is grafted to make the connection between artery and vein. Over time, it will be possible to put two dialysis needles into the enlarged vein or graft. This allows blood to be removed from the body through one needle, passed through the HD machine and returned to the patient via the second needle.
Peritoneal dialysis (PD)
PD uses the body’s own potential peritoneal cavity and peritoneal membrane as an internal dialysis system. Patients dialyse at home (under the care of specialist renal nurses) by instilling clean dialysis fluid (dialysate) into this cavity via a specialist abdominal catheter. Over a set period of time, toxins, waste products and excess fluid move from the patient’s blood into the dialysate via osmosis, diffusion and convection. When the prescribed time period has elapsed, the now waste- filled dialysate is drained away and replaced with fresh dialysate. This fluid ‘exchange’ process can either be performed manually (continuous ambulatory peritoneal dialysis, CAPD) or by machine (automated peritoneal dialysis, APD).
Transplantation
This is the donation of a healthy kidney from either a living or deceased donor to another person with little or no kidney function. Bearing in mind the serious comorbidities associated with CKD, not all patients will be healthy enough to undergo transplant surgery. Around one in three people with kidney failure is suitable for a transplant. Transplantation may occur before the need for dialysis has arrived or after the point where dialysis is established. Powerful anti-rejection medication must be taken for life to prevent the kidney being rejected by the recipient’s immune system (NHS, 2022b).
Conservative management
Conservative medical management focuses on providing kidney supportive care to promote quality of life without pursuing dialysis or transplantation. Treatment relies on the patient following dietary and medication controls as an outpatient. The aim is to preserve renal function, manage symptoms and enhance quality of life for as long as possible, but the overall decline cannot be stopped. Conservative management is best delivered through a multidisciplinary collaboration between the patient, family and primary, secondary and tertiary services (Schell et al, 2023).
In the author’s clinical experience, some patients may feel that dialysis is too heavy a burden in light of their age, frailty, or comorbidities and will choose to have their symptoms managed in this way. Also, in some cases, initiating dialysis would not necessarily lead to any longer survival.
WHO SHOULD BE TESTED FOR CKD?
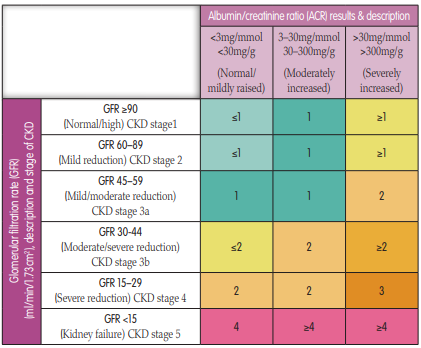
To identify CKD at the earliest opportunity, NICE (2021a) recommends that patients in the following high-risk groups should have their GFR and ACR screened. (Figure 2):- Diabetes — the largest cause of CKD and GFR testing at least annually is recommended. This would need to be increased if the patient’s CKD shows progression. Good glycaemic control is essential
- Hypertension — patients may be unaware of the link between CKD and high blood pressure and home blood pressure monitoring is useful. ACE inhibitors (ACEis) or angiotensin receptor blockers (ARBs) are often the initial antihypertensive drugs of choice:
- For those diagnosed with CKD, aim to keep the systolic blood pressure below 140mmHg (target range 120–139mmHg) and the diastolic blood pressure below 90mmHg. For patients with CKD and diabetes, and those with an ACR of 70mg/mmol or more, aim to keep the systolic blood pressure below 130mmHg (target range 120–129mmHg) and the diastolic blood pressure below 80mmHg
- Salt restriction and guidance on healthy diet and smoking cessation would be appropriate
- Patients who have experienced AKI
- CVD (ischaemic heart disease, chronic heart failure, peripheral vascular disease or cerebral vascular disease)
- Structural renal tract disease, recurrent renal calculi or prostatic hypertrophy
- Multisystem diseases with potential kidney involvement, e.g. systemic lupus
- Family history of end-stage kidney disease (CKD stage 5) or hereditary kidney disease, e.g. polycystic kidney disease
- Opportunistic detection of haematuria or proteinuria (as above, protein in the urine must not be assumed to relate only to a UTI)
- Patients on certain medications:
- NICE (2021a) also recommended that the GFR should be checked at least annually in people prescribed drugs known to be nephrotoxic, such as calcineurin inhibitors (for example, cyclosporin or tacrolimus), lithium and long-term doses of non-steroidal anti-inflammatory drugs
- Metformin should be avoided in CKD stages G4 and G5 (UKKA, 2017)
- Should the patient have albuminuria and is started on an ACEi or ARB, it is important to check that their potassium level is in range before, and two weeks after, to assess for any worsening renal function.
FIGURE 2.
The recommended number of times per year that the GFR should be checked for every CKD patient and also for those at risk of developing CKD. Based upon GFR and ACR results (adapted from NICE, 2021a).WHO SHOULD BE REFERRED TO A NEPHROLOGIST?
Most CKD patients can be managed effectively within the community. NICE guidelines (2021a) recommend early referral of certain patients to nephrologists to preserve residual renal function and slow disease progression.
Those in the following groups definitely require specialist assessment but will benefit greatly from ongoing support from primary care nurses and a shared-care approach:
- A symptomatic patient with a GFR less than 30ml/min/1.73 m2 , with or without diabetes. This would not include those patients receiving end-of-life care
- ACR 70mg/mmol or more, unless known to be caused by diabetes and already appropriately treated
- ACR 30mg/mmol or more (ACR category A3), together with haematuria (a possibility of glomerulonephritis)
- A sustained decrease in GFR of 25% or more, and a change in GFR category or sustained decrease in GFR of 15ml/ min/1.73 m2 or more within 12 months. NICE (2021a) recommended that a minimum of three GFR estimations should be obtained over a period of not less than 90 days to establish this. If the patient has a low baseline GFR, repeat the GFR within two weeks to exclude causes of acute deterioration of renal function, e.g. AKI or recently commencing on anti-hypertensives, such as ACEis, ARBs or a direct renin inhibitor
- Patients with hypertension that remain poorly controlled despite the use of at least four antihypertensive drugs at therapeutic doses
- Known or suspected rare or genetic causes of CKD
- Suspected renal artery stenosis (NICE, 2021a).
It is crucial for nephrologists to investigate and diagnose the cause of CKD in patients referred to them. Specific treatments are often tailored based on the underlying condition contributing to CKD. In addition to addressing the specific cause, nephrologists commonly recommend treatments aimed at slowing the progression of CKD. These may include:
- The use of ACE inhibitors and ARBs which have been shown to slow the progression of CKD
- The removal and avoidance of nephrotoxic medications
- Increasingly, the use of sodium-glucose cotransporter-2 inhibitors (SGLT2 inhibitors). These are oral treatments used in patients with and without diabetes to slow the progression of CKD. SGLT2 inhibitors have shown efficacy in reducing the risk of kidney failure and cardiovascular events in patients with CKD (NICE, 2021b).
FREQUENCY OF REVIEWS
Once a baseline GFR and ACR have been established, NICE then provides further guidance as to the frequency of reviews for CKD patients (Figure 2).These reviews offer the chance to assess and manage the patient’s CKD and CV risks. During these reviews, primary care nurses can detect early CKD and monitor the progression of established renal disease. Basic monitoring should include:
- Blood tests for renal function (serum creatinine, urea, sodium and potassium), GFR, full blood count (FBC), cholesterol and glucose
- Urine test for ACR
- Assess for symptoms of CKD, e.g. oedema, anaemia
- Blood pressure and weight. Both reduced eGFR and albuminuria are markers of cardiovascular risk which should be documented. However, the most important aspect of management is blood pressure control
- Medication review to check for nephrotoxic drugs such as the long-term use of NSAIDs. Always ensure that the patient is prescribed the correct renal dose in line with the eGFR for all new and existing medications
- Advice can also be offered regarding diet, lifestyle, alcohol intake and smoking cessation.
CONCLUSION
CKD has an impact not only on every system of the body, but also the individual’s quality of life. The complications of renal disease lead to greater morbidity, mortality and financial cost. Early detection of CKD in high-risk groups is key, potentially reducing the risk of complications and even delaying progression by following a controlled plan of management and interventions. Primary care nurses are experienced members of the MDT with the skills required to identify, manage and support this patient group. Factors linked to the progression of CKD are frequently the same as those of CVD. By continuing to target the modifiable risk factors in both groups, Primary care nurses will not only be instrumental in reducing CVD in patients with CKD but will also slow or potentially prevent renal disease reaching stage 5.The data reported here has been supplied by the UK Renal Registry (UKRR) of the UK Kidney Association. The interpretation and reporting of these data are the responsibility of the author and should not be seen as an official policy or interpretation of the UKRR or the UK Kidney Association.
REFERENCES
Bailey M, Unwin R (2024) Renal physiology. In: Johnson R, Floege J, Tonelli M, eds. Comprehensive Clinical Nephrology. 7th edn. Elsevier, Philadelphia: chap 2Blaine J, Chonchol M, Levi M (2015) Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 10(7): 1257–72
Carter J, Tomson C, Stevens P, Lamb E (2006) Does urinary tract infection cause proteinuria or microalbuminuria? A systematic review. Nephrol Dial Transplant 21(11): 3031–37
Catley C (2016) Chronic kidney disease: detection and management for GPNs. J General Practice Nurs 2(3): 42–50
Dring B, Hipkiss V (2015) Managing and treating chronic kidney disease. Nurs Times 111(7): 16–19
Hasmi MF, Onecia B, Lappin SL (2023) End-stage renal disease. StatPearls. Available online: www.ncbi.nlm.nih.gov/books/NBK499861/
Kidney Disease Improving Global Outcomes (2013) 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Supplements 3(1): 1–163
Kidney Care UK (2023) Key Facts about Kidneys. Available online: https://kidneycareuk.org/kidney-disease-information/about-kidney-health/facts-about-kidneys/
Kidney Research UK (2023) Kidney disease: A UK public health emergency. The health economics of kidney disease to 2033. Available online: www.kidneyresearchuk.org/wp-content/uploads/2023/06/Economics-of-Kidney-Disease-full-report_accessible.pdf
Kriz W, Elger M (2015) Renal anatomy. In: Johnson R, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th edn. Elsevier, Philadelphia: chap 1
Lau WL, Festing MH, Giachelli CM (2010) Phosphate and vascular calcification: emerging role of the sodium-dependent phosphate cotransporter PiT-1. Thromb Haemost 104(3): 464–70
Major RW, Shepherd D, Medcalf JF, Xu G, Gray LJ, et al (2020) Correction: The Kidney Failure Risk Equation for prediction of end stage renal disease in UK primary care: An external validation and clinical impact projection cohort study. PLOS Medicine 17(7): e1003313
Mahmoodi BK, Gansevoort RT, Veeger NJ, Matthews AG, Navis G, Hillege HL, van der Meer J, Prevention of Renal and Vascular End-stage Disease (PREVEND) Study Group (2009) Microalbuminuria and risk of venous thromboembolism. JAMA 301(17): 1790–7
NHS (2022a) Urine albumin to creatinine ratio (ACR). Available online: www.nhs.uk/conditions/acr-test/
NHS (2022b) Kidney transplant. Available online: www.nhs.uk/conditions/kidney-transplant
National Institute for Health and Care Excellence (2021a) Chronic kidney disease in adults: assessment and management. CG182. NICE, London. Available online: www.nice.org.uk/guidance/ng203/chapter/Recommendations#investigations-for-chronic-kidney-disease
National Institute for Health and Care Excellence (2021b) NICE recommend dapagliflozin for people with chronic kidney disease. NICE, London. Available online: www.nice.org.uk/news/article/nice-recommend-dapagliflozin-for-people-with-chronic-kidney-disease
National Institute for Health and Care Excellence (2022) Roxadustat for treating symptomatic anaemia in chronic kidney disease. Technology appraisal guidance. TA807. NICE, London. Available online: www.nice.org.uk/guidance/ta807
National Institute for Health and Care Excellence (2023) Finerenone for treating chronic kidney disease in type 2 diabetes. Technology appraisal guidance TA877 Available online: www.nice.org.uk/guidance/ta877/chapter/1-Recommendations
O’Lone EL, Hodson EM, Nistor I, Bolignano D, Webster AC, Craig JC (2019) Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database of Syst Rev Issue 2. Art. No: CD007857
Schell J, Arnold R (2023) Kidney palliative care: Conservative kidney management. Available online: www.uptodate.com/contents/kidney-palliative-care-principles-benefits-and-core-components?search=kidney%20palliative%20care&source=search_result&selectedTitle=1~18&usage_type=default&display_rank=1
UK Kidney Association (2017) The UK eCKD Guide. Available online: https://ukkidney.org/health-professionals/information-resources/uk-eckd-guide
UK Renal Registry (2023) UK Renal Registry 25th Annual Report – data to 31/12/2021, Bristol, UK. Available online: https://ukkidney.org/audit-research/annual-report
Wish B (2006) Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 1(Supplement 1): S4–8
Christine Catley is renal research nurse, Broomfield Hospital, Mid and South Essex NHS Foundation Trust.
This piece was first published in the Journal of General Practice Nursing. To cite this article use: Catley C (2024) Chronic kidney disease: detection and management for GPNs. J Gen Practice Nurs 10(2): 50–58